Serine Repeat Antigen 5 (SERA5) and Egress
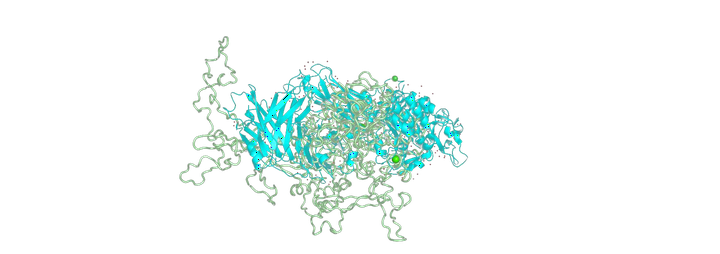 Schrodinger protein docking output
Schrodinger protein docking outputMalaria remains a challenging disease even in the 21st century. In the complex Plasmodium falciparuml life cycle, the asexual erythrocytic stage of merozoite is critical, during which erythrocyte invasion and egress materialize.
Egress from erythrocyte is protease-dependent and Serine Repeat Antigens (SERA) SERA5 and SERA-6 are the most highly expressed merozoite catalytic proteases that help in releasing the parasite during schizont rupture. Both SERA 5 and SERA 6 are processed by a Subtilisin like protease - SUB 1. Recent reports show that SERA 5 is a pseudo protease, with a critical regulatory role, whereas SERA 6 contributes to membrane rupture and release of denovo merozoites from the host erythrocyte. Earlier observations also show that parasites lacking SERA5 undergo accelerated but defective egress in which the bounding vacuole and red blood cell membranes do not rupture properly while in SERA6-null parasites RBCM rupture does not occur. It is hypothesized that SERA 5 acts as an abundant SUB-1 substrate, competing with the SUB-1 mediated cleavage of other key substrates including SERA 6, thus slowing down the kinetics of egress and acting as a negative regulator.
In this work, using computational approaches, this hypothesis was evaluated, to understand the relative binding and affinity of SUB 1 to its substrates SERA 5 and SERA 6 for their processing. Using the PDB structures available in databases for SERA5 (protease domain) and SUB1 along with the ROBETTA generated structures of SERA 6 and full-length SERA 5, several protein docking simulations using the Schrödinger platform were done to predict the relative binding and processing. The timeline of egress has also been used to sketch and explain the possible mechanism for their interaction and sequential action. Our docking results showed that SERA6 has a relatively higher binding affinity to SUB1 compared to SERA 5. However, SERA5 has a much higher expression than SERA6 during the process of egress. So, SERA 5 competes with SERA 6 leading to inhibition of its processing by SUB 1. SERA 5’s role as a negative kinetic regulator could only be a function of the higher expression and abundance of SERA 5 within the erythrocytes and not higher affinity to SUB-1. Thus, SUB 1 is being used up to interact with SERA5, thus delaying the processing of SERA 6, and causing time lag in the kinetics of egress.